How it works:
Our Design & Manufacturing Solutions

Quite simply if it can be built we can build it. From surgical implantables (screws, fixation systems), devices with electronics, custom software development, complex assemblies and sub-assemblies, sterilization, complex materials, and injection molding.
Pro-Dex is a big step ahead of the pack. From our location, to our industry experience and comprehensive suite of services we lead the contract manufacturing industry for good reason.
510(k) filings to device history files let us manage your documentation needs.
Our facilities are registered with the FDA and CA Department of Public Health Food & Drug Branch.
We have extensive experience precision manufacturing for the world’s largest companies.
Pro-Dex was established in 1978 and has delivered over 50,000 powered devices.
Our assembly model utilizes cells with extensive cross training and testing.
Standalone service. Let us design your testing protocols. Quality is our DNA.
Pro-dex is a public company and trades under the symbol “PDEX

How it works:
Our Design & Manufacturing Solutions
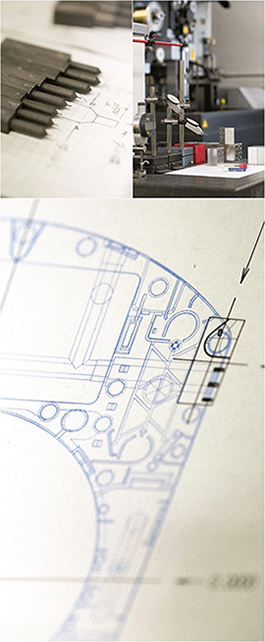
Prodex offers turnkey manufacturing solutions for a wide variety of industries. From prototyping to engineering to manufacturing and assembly to validating your suppliers and supply chain management to meeting regulatory and compliance needs we have solutions for every aspect of your project and manufacturing needs. Bringing products to market is our specialty. Schedule a facility visit today!
We invite you to come see our six phase development process in action; we begin with proof of design and product feasibility testing, leveraging our in house engineering talent and long history of manufacturing experience. We then develop designs for manufacturability and concurrently develop processes for manufacture and assembly. Prior to product transfer and release all of our products go through a rigorous verification and validation process. Finally we conduct post production evaluations, continually optimizing and making process improvements.

2. Manufacture. Our versatile high precision manufacturing facilities are able to produce on a low volume quick turnaround basis as well as on a high volume cost effective basis. From high precision injection molding and tooling to ultrasonic welding or precision motion control to machining at extremely tight tolerances we excel at speed to market, high quality, on-time deliveries and reduced manufacturing costs. Our engineering team will work to help every step of the way, working hard to avoid problems before they happen and suggesting alternatives to reduce the cost of tools, use of parts, and optimize the manufacturing so that it is lean and well within your specifications. We are one of only a few local FDA registered and ISO 13485 certified facilities with decades of experience manufacturing finished medical devices. Our employee base is extremely loyal with five of our key machine shop employees having almost a century of experience between them.

3.Assembly. Our assembly facilitiy is equally versatile. We assemble in stages with regular testing intervals to increase efficiency and virtually eliminate any fully assembled performance failures. Testing is built into the culture of our assembly team. Open channels of communication between our inventory, manufacturing and assembly teams with strong management oversight allows for a culture of continuous improvement. Assembly is an important aspect of our turnkey product/compent concept to market strategy. We aren’t simply a contract manufacturer. We are capable of driving your project from beginning to end.

4. Testing. Rigorous testing is a key aspect of our Quality Management System. We are so recognized for the quality of our testing that some customers outsource their finished product testing to our facilities for our team to test on their behalf. Our engineering is involved every step of the way, monitoring testing outcomes and constantly looking for product design enhancements. Our product testing capabilities serve as a standalone capability for Pro-dex and are another way that we set ourselves apart from the pack, offering solutions at every stop of the manufacturing process.

5. Documentation & Compliance. These are critical aspects of the manufacturing in many industries. In the manufacture of medical devices manufacturers are required to file 510(k) Clearances notifying the intent to market a medical device. Quality System Regulations, Design History Files, and strong and effective documentation controls are essential to proper development and manufacturing. With Pro-dex you can outsource your documentation and compliance needs or leverage the expertise of our in house team. We have been qualified by some of the largest medical device distributors in the world and are more than capable of handling your documentation and filing requirements.
6. Delivery. Pro-dex offers multi-directional logistics and fulfillment. We not only drop ship finished and laser etched products globally but we receive finished devices for repairs and refurbishments. Our job is to not only manage every aspect of the product design, manufacture, assembly, and testing, but to also provide the backup and repair services needed for you and your customers to use our products with the peace of mind that we can service them. Our ERP system keeps us on top of inventory and delivery times and ensures that we deliver your products exactly when you need them.
Pro-Dex is a big step ahead of the pack. From our locations, to our industry experience and comprehensive suite of services we lead the contract manufacturing industry for good reason.

Prototyping & Validation
We have seen a lot of product ideas and can help you successfully develop your concept for manufacture.
Experience drawing detailed design specifications with hardware / software interfaces, tolerance analyses & manufacturing feasibility.
Experts in embedded systems and solutions (battery powered, torque limiting software, led displays, etc).
Specialize in transforming ideas and concepts into functional prototypes ready for manufacturing.
Decades of experience with tight tolerances and flexible & high mix manufacturing.
At our regularly audited ISO 13485 certified facilities good manufacturing practices are embedded in our culture.
Circuit and PCB design, programming (C++, Java, Visual Basic, etc), display sub-systems for instrumentation.
Still not sure how our business works or what our manufacturing capabilities are? Don’t hesitate to contact us! We pride ourselves at being extremely responsive and chances are that our engineering team has already built what you need.
Corporate
2361 McGaw Avenue, Irvine,CA 92614
Manufacturing
14401 Franklin Avenue, Tustin,CA 92780
1-800-562-6204
sales@pro-dex.com